Can we pool the samples to reduce the cost of the analysis?.
The answer is not easy, it is clear that, if we take samples of semen or saliva, the concentration of the virus in the first post-infection days will be very low and we will further decrease the accuracy and sensitivity of the sampling, so some authors recommend not pooling this type of samples. On the other hand, in blood samples, whether collected with vacutainer or with swab, in addition to having an early presence of viremia, they will also have a higher viral load, so they are valid for the realization of pool. In any case, the number of samples to be mixed in a pool should not be greater than 3-5. We must always think of trinomial practicality-cost-benefit. It is clear that making a pool of 5 blood samples will be the most practical and will help us to reduce costs, but we have to take into account that we will lose sensitivity in detection (Table 1).
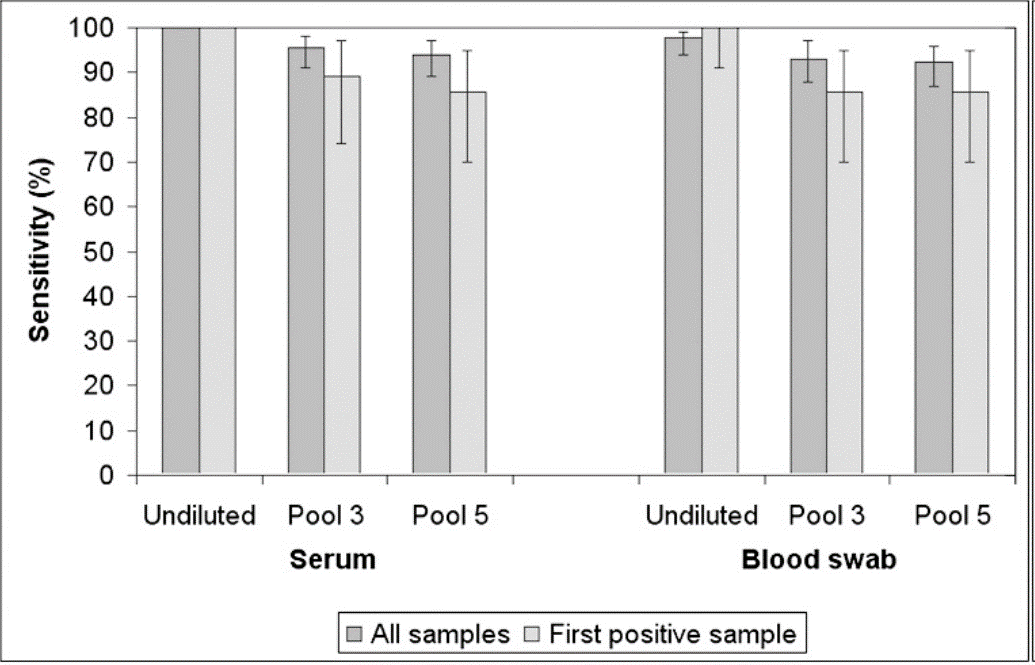
Table 1: effect of pooling on the sensitivity of RT-PCR.
Source: Rovira et al. J. Vet. Diagn. Invest. (2007)
Once we have determined all the parameters, type of sample, sampling method, pool performance or not, it is necessary to determine the frequency of testing of the animals. For this, we can rely on mathematical models that will determine the detection rate of the different test percentages according to the frequency and number of animals. For this there are different virtual calculators with which, adding the census of our center, the type of sample we use and the frequency of testing will give us the probability of detection of the entry of the virus into the farm (Picture 1).
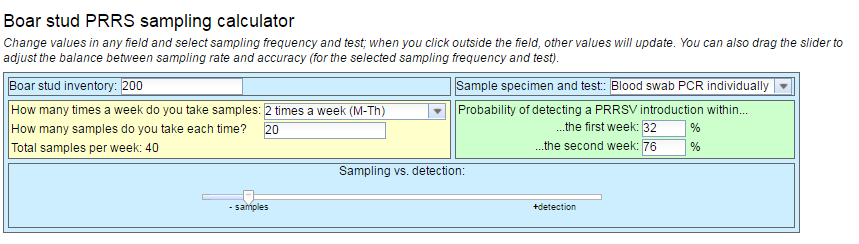
Picture 1: calculator
Source: AASV 2018
In order to achieve maximum safety in the center, the boars should be tested with blood samples in PCR in each extraction. In this way we can maximize the sensitivity of the test to 100%, but we understand that in many cases it is not practical at field level due to time, cost, or rejection of animals, so we have to decide what level of safety and risk we accept on our farm to calculate the number of animals to be tested. It will also necessary to decide if we want to do this calculation for each day of extraction or at the weekly, monthly level, etc. because that will change the costs. What does seem clear is that the boars of maternal lines should be tested as often as possible, since the economic effect of the dissemination of the virus can become much greater than that of the finishers.
In Table 2, we can see a simulation in a center of 200 boars, between the probability of detection in the first and second week after infection and the cost of carrying out the test as the number of samples increases.
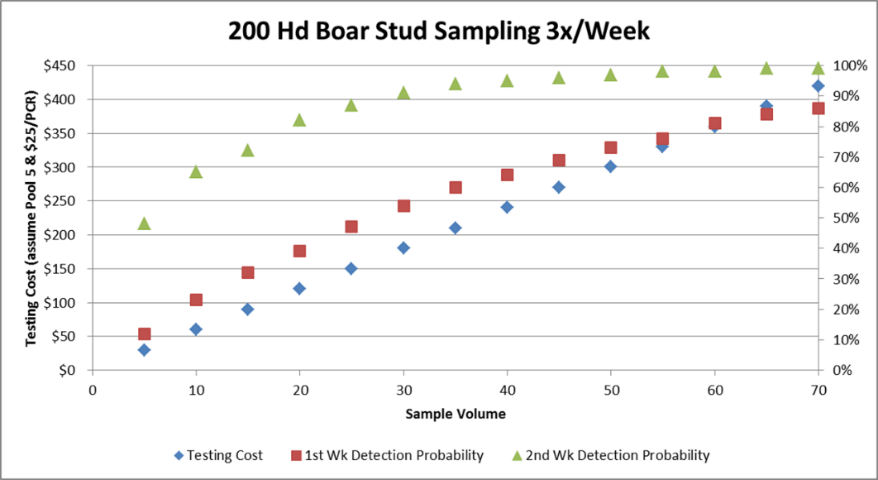
Table 2: Clayton Johnson, Carthage Veterinary Services. ITM 2017
Regarding the associated cost per dose, it is difficult to give a monetary value, since this will depend on multiple factors, such as the availability of laboratories in our area, number of samples and type of test to be performed, how efficient we are when producing (obtaining a higher number of doses per ejaculate will decrease the associated cost).
In conclusion, there are multiple strategies to monitor/diagnose the entry of PRRS in a boar stud. Each of them provides greater or lesser sensitivity and carries a greater or lower cost, depending on the way of work, the use of animals (internal use vs sales of doses), and the risk of virus entering into the CIA, so it is important that all production strata and workers are aware of the importance of maintaining biosecurity and performing proper monitoring to avoid problems.
BIBLIOGRAPHY
Guérin B., Pozzi N. Viruses in boar semen: detection and clinical as well as epidemiological consequences regarding disease transmission by artificial insemination. Theriogenology. 2005 Jan 15; 63(2):556-72.
Pepin B.J., Kittawornrat A., Liu F., Gauger P.C., Harmon K., Abate S., Main R., Garton C., Hargrove J., Rademacher C., Ramirez A., Zimmerman J. Comparison of specimens for detection of porcine reproductive and respiratory syndrome virus infection in boar studs. Transbound Emerg Dis. 2015 Jun; 62(3):295-304.
Reicks, D. L., C. Munoz-Zanzi, and K. Rossow, 2006b: Sampling of adult boars during early infection with porcine reproductive and respiratory syndrome virus for testing by polymerase chain reaction using a new blood collection technique (bloodswab method). J. Swine Health Prod. 14, 258–264.
Reicks D., Muñoz-Zanzi C., Mengeling W., Christopher-Hennings J., Lager K., Polson D., Dee S., Rossow K. Detection of porcine reproductive and respiratory syndrome virus in semen and serum of boars during the first six days after inoculation. J Swine Health Prod. 2005; 14:35–41.
Rovira, A., T. Clement, J. Christopher-Hennings, B. Thompson, M. Engle, D. Reicks, and C. Munoz-Zanzi, 2007a: Evaluation of the sensitivity of reverse-transcription polymerase chain reaction to detect porcine reproductive and respiratory syndrome virus on individual and pooled samples from boars. J. Vet. Diagn. Invest. 19, 502–509.
https://www.3tres3.com/articulos/limitaciones-de-la-pcr-en-el-diagnostico-del-prrs_40023/
https://www.3tres3.com/articulos/retos-en-el-diagnostico-de-prrsv_35536/